Frequently Asked Questions
Transcranial Magnetic Stimulation (TMS) is a non-invasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression.
TMS therapy targets specific areas of the brain involved in mood regulation. Magnetic pulses stimulate neurons, enhancing their activity and improving mood.
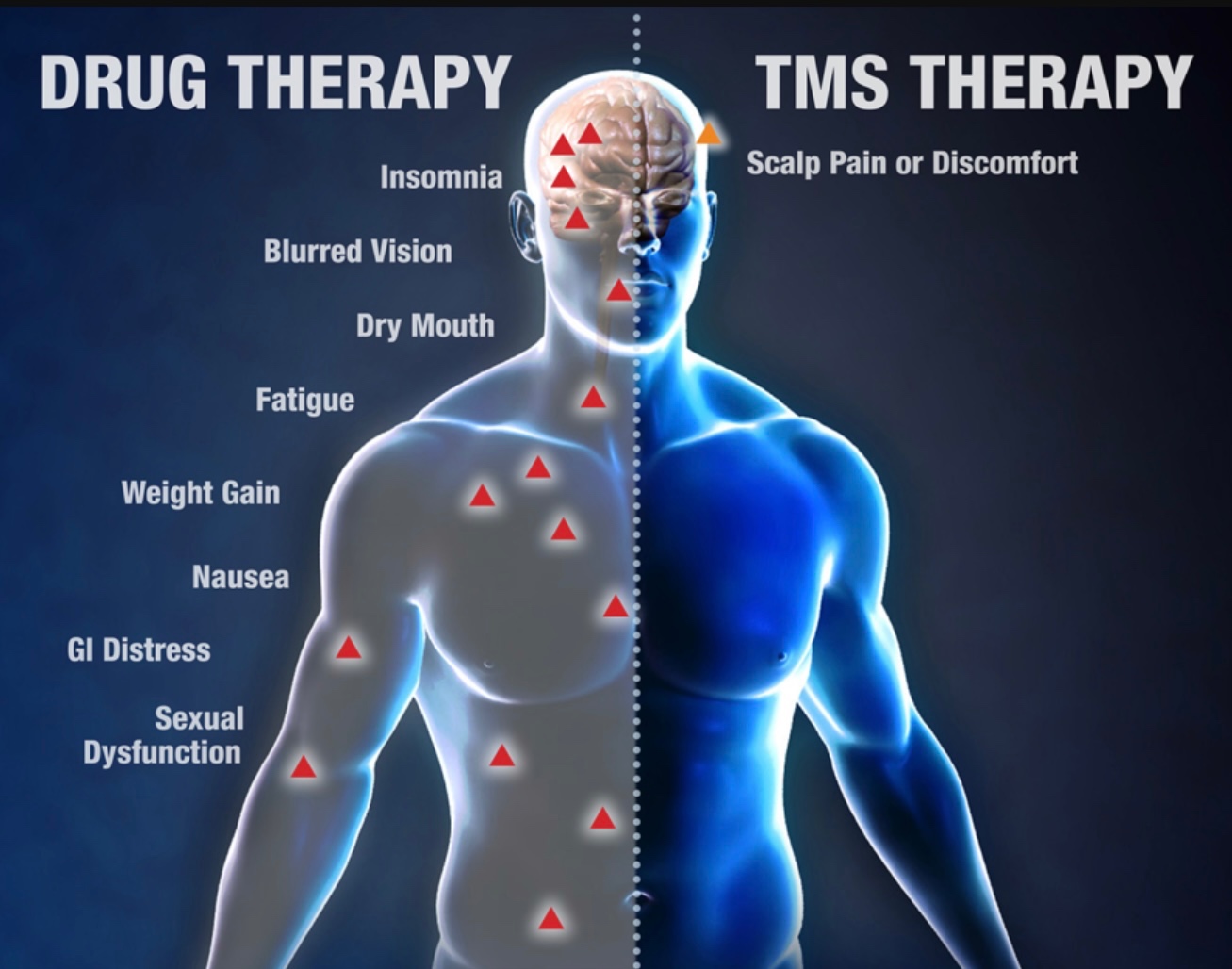
Yes, TMS is considered safe and well-tolerated. It is FDA-cleared for the treatment of major depressive disorder and has a favorable side effect profile compared to traditional medications.
TMS is particularly beneficial for individuals who have not responded well to antidepressant medications or who experience intolerable side effects from them.
Each TMS session typically lasts between 20 to 40 minutes. Patients usually undergo treatment five days a week for seven weeks.
The most common side effect is mild scalp discomfort or headache at the treatment site, which usually resolves after the first few sessions. TMS does not have the systemic side effects commonly associated with antidepressants.
Many patients begin to notice improvements in their symptoms within the first few weeks of treatment. However, individual responses can vary.
Most major insurance companies cover TMS therapy for the treatment of major depressive disorder, but coverage can vary. It’s best to check with your insurance provider for specific details regarding coverage.
This depends on your specific situation and should be discussed with your healthcare provider. Some patients may continue taking their medications while receiving TMS, while others may not.
TMS is FDA-cleared for the treatment of major depressive disorder, but research is ongoing into its effectiveness for other conditions, such as anxiety, OCD, and PTSD.

Medical Professionals
808080+
Adipiscing elit, do eiusm.
Effective Techniques
8080
Sed eiusmod tempor.

