Transcranial Magnetic Stimulation (TMS) has emerged as a groundbreaking non-invasive treatment for major depressive disorder (MDD) and other mental health conditions. This article explores the key aspects of TMS, its effectiveness, and its place in modern mental health care. This article covers the following topics:
- What is TMS?
- How Effective Is TMS Compared to Antidepressants?
- The Area of the Brain TMS Targets
- New Understanding of Major Depressive Disorder: From Chemical Imbalance to Neural Connectivity
- TMS Impact on Key Neural Networks
- Different Forms of TMS
- Summary of Research on TMS Benefits
- Safety of TMS
- Typical Patient Experience of TMS
- Conclusion

1. What is TMS?
TMS is a non-invasive procedure that uses magnetic fields to stimulate nerve cells/neurons in specific areas of the brain. It is primarily used to treat depression, especially in individuals who have not responded to conventional treatments such as medication or therapy.
During a TMS session, an electromagnetic coil is placed on the scalp, generating magnetic pulses that penetrate the skull and activate targeted brain regions. Unlike electroconvulsive therapy (ECT), TMS does not involve anesthesia or cause seizures, making it a more tolerable option for many patients.
What is Neuroplasticity?
Neuroplasticity is the brains ability to change its structure and function in response to learning and experience
TMS can improve neuroplasticity in your brain through increasing neural activity. It can also increase white matter integrity and gray matter volume and thickness.
2. How Effective is TMS Compared to Antidepressants
TMS offers a compelling alternative for individuals with depression who have not achieved adequate relief from antidepressants. Studies show that up to 60-80% of patients experience significant improvement in symptoms, while around 60-70% achieve full remission after TMS treatment. This contrasts with antidepressants, where only about 30% of patients achieve remission on their first medication trial.
TMS effectiveness often surpasses antidepressants in treatment-resistant depression, offering hope for those who have not responded to multiple medication regimens. Additionally, TMS has fewer systemic side effects (e.g., no weight gain, sexual dysfunction, or drowsiness), making it a preferable choice for some patients.

The Area of the Brain TMS Targets
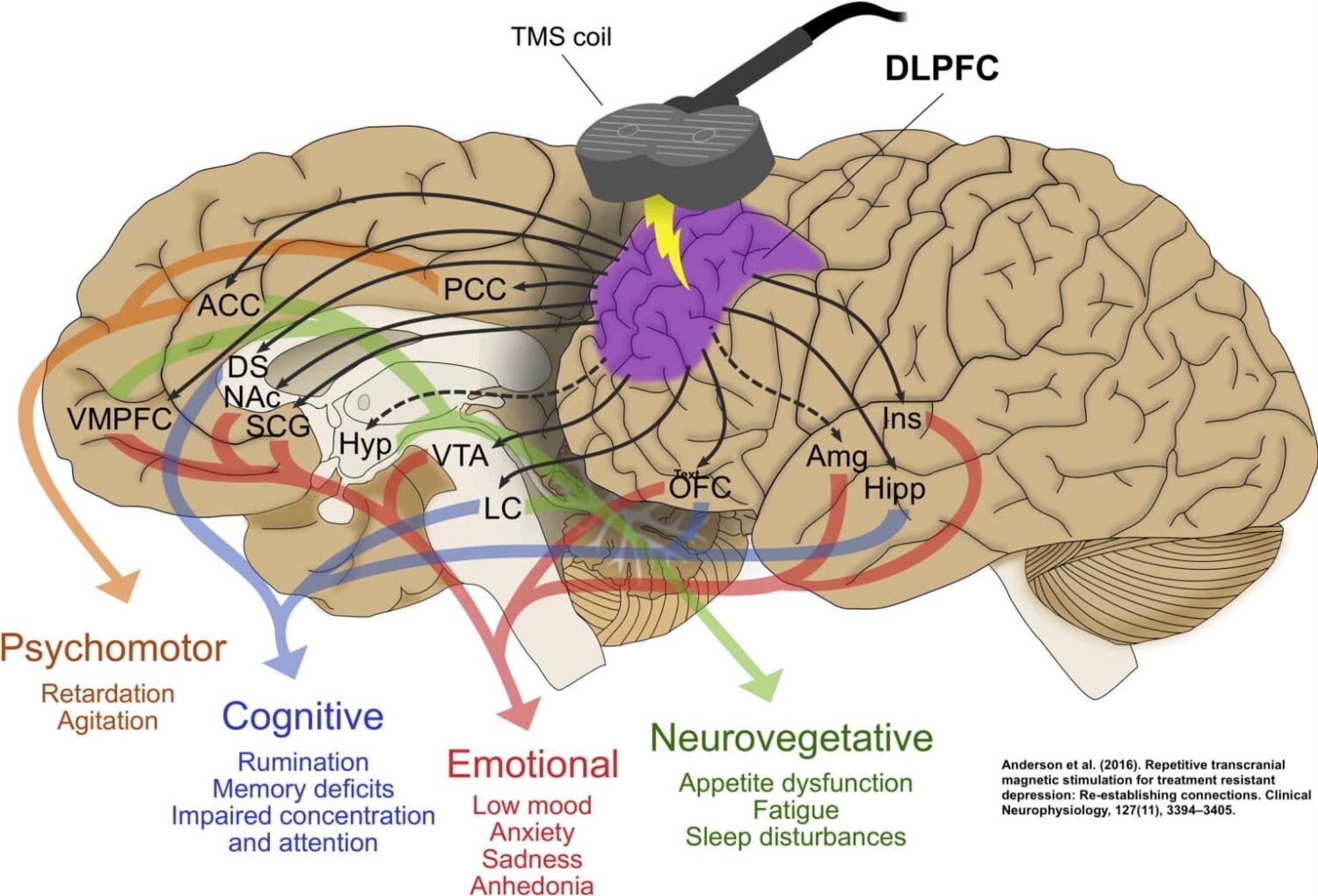
TMS primarily targets the dorsolateral prefrontal cortex (DLPFC)—a region involved in mood regulation, executive functioning, and emotional control. The left DLPFC is often underactive in patients with depression, while the right DLPFC may be overactive.
How TMS Works:
- Left DLPFC: High-frequency stimulation to increase activity in this underactive area.
- Right DLPFC: Low-frequency stimulation to reduce overactivity.


New Understanding of Major Depression: From Chemical Imbalance to Neural Connectivity
The traditional understanding of major depressive disorder (MDD) has long focused on the “chemical imbalance” theory, emphasizing deficits in neurotransmitters such as serotonin, dopamine, and norepinephrine. While these chemical aspects play a role, emerging research reveals that MDD is more accurately characterized by disruptions in the connectivity and functioning of neural networks and pathways within the brain. This paradigm shift has profound implications for treatments like TMS.
From Chemical Imbalance to Network Dysregulation
Modern neuroscience views depression as a disorder of dysregulated brain networks rather than simply a lack of neurotransmitters. Here’s how this new understanding has developed:
Neural Connectivity:
- In depression, key brain regions, including the prefrontal cortex, limbic system, and subcortical structures, show disrupted communication and synchronization.
- For example, overactivation of the amygdala (associated with emotional reactivity) and underactivation of the prefrontal cortex (responsible for cognitive control) create an imbalance that perpetuates negative emotional states.
Functional Connectivity of Neural Networks:
- Research using functional MRI (fMRI) has highlighted altered connectivity in networks like the Default Mode Network (DMN), Salience Network (SN), and Central Executive Network (CEN).
- These changes lead to core symptoms of depression, such as rumination, emotional dysregulation, and cognitive impairments.
Pathways Over Chemical Focus:
- While serotonin and other neurotransmitters remain essential, their role is now understood in the context of modulating the activity and connectivity of these networks.
- The disruption of pathways connecting the DLPFC with deeper structures like the hippocampus and amygdala is a primary target for innovative treatments like TMS.
TMS and the Connectivity Model
TMS fits seamlessly into this new understanding by directly influencing network connectivity rather than solely targeting neurotransmitter levels. Here’s how TMS works within this framework:
Restoring Neural Balance:
- By stimulating the dorsolateral prefrontal cortex (DLPFC), TMS modulates connectivity with other brain regions, helping recalibrate overactive or underactive networks.
Targeting Dysregulated Networks:
- TMS reduces the overactivity of the DMN, improving a patient’s ability to disengage from negative self-focus and rumination.
- It strengthens the functional communication between the DLPFC and regions of the SN and CEN, restoring cognitive control and emotional regulation.
A Personalized Approach:
- Advanced imaging techniques (e.g., fMRI-guided TMS) allow clinicians to personalize TMS treatments by identifying the unique connectivity patterns contributing to a patient’s depression.
Broader Implications for Depression Treatment
 This shift from a chemical imbalance to a connectivity-focused understanding has opened new avenues for therapeutic innovation:
This shift from a chemical imbalance to a connectivity-focused understanding has opened new avenues for therapeutic innovation:
- Non-invasive neuromodulation techniques like TMS directly address network dysfunctions without the systemic side effects of medications.
- It also explains why some patients with MDD may not respond to antidepressants: their underlying issues may lie more in network dysregulation than in neurotransmitter imbalances.
As the connectivity model continues to evolve, treatments like TMS are at the forefront of providing more effective and personalized care for individuals with major depressive disorder. This approach not only alleviates symptoms but also improves the underlying brain dynamics that support emotional and cognitive well-being.
TMS Impact on Key Neural Networks
 In addition to directly targeting the dorsolateral prefrontal cortex (DLPFC), Transcranial Magnetic Stimulation (TMS) influences several interconnected neural networks that play critical roles in mood regulation, cognitive function, and emotional processing. The three most relevant networks affected by TMS are the Default Mode Network (DMN), the Salience Network (SN), and the Central Executive Network (CEN).
In addition to directly targeting the dorsolateral prefrontal cortex (DLPFC), Transcranial Magnetic Stimulation (TMS) influences several interconnected neural networks that play critical roles in mood regulation, cognitive function, and emotional processing. The three most relevant networks affected by TMS are the Default Mode Network (DMN), the Salience Network (SN), and the Central Executive Network (CEN).
Default Mode Network (DMN)
The Default Mode Network is active during rest and self-referential thinking, such as daydreaming, introspection, or rumination. In individuals with depression, the DMN is often hyperactive, contributing to excessive negative self-focus and rumination.
- TMS Impact on the DMN:
- High-frequency stimulation of the left DLPFC disrupts the overactivity of the DMN, reducing rumination and improving emotional regulation.
- This recalibration helps patients shift focus from self-critical thoughts to more adaptive cognitive processes, facilitating recovery from depressive symptoms.
Salience Network (SN)
The Salience Network detects and filters relevant stimuli, determining what requires attention. It plays a central role in emotional regulation and switching between the DMN and the CEN. Dysfunction in the SN is associated with difficulties in emotional processing and heightened sensitivity to negative stimuli, common in depression.
- TMS Impact on the SN:
- TMS enhances SN functioning by improving communication between the DLPFC and deeper brain structures like the anterior cingulate cortex (ACC), (which is associated with our will power) and insula.
- This leads to better regulation of emotional salience, helping individuals focus on positive or neutral stimuli rather than overemphasizing negative experiences.
Central Executive Network (CEN)
The Central Executive Network is involved in higher-order cognitive processes, including working memory, decision-making, and goal-oriented behavior. Depression often results in underactivity of the CEN, leading to poor focus, indecisiveness, and diminished problem-solving abilities.
- TMS Impact on the CEN:
- By stimulating the left DLPFC, TMS increases the activity and connectivity within the CEN, enhancing cognitive control and executive functioning.
- Patients often report improvements in concentration, task efficiency, and the ability to plan and execute actions.

The Interplay Between Networks
TMS promotes a healthy balance and communication between these networks, enabling:
- Reduced overactivity of the DMN (less rumination).
- Improved salience processing via the SN (better emotional regulation).
- Enhanced cognitive control through the CEN (greater focus and decision-making).
This integrated effect is one of the reasons TMS is effective not only in alleviating depressive symptoms but also in addressing the cognitive and emotional challenges that often accompany depression.
Different Forms of TMS
There are several variations of TMS, tailored to meet different clinical needs, these include the following forms of TMS:
- Repetitive Transcranial Magnetic Stimulation (rTMS)
- Intermittent Theta Burst Stimulation (iTBS)
- Continuous Theta Burst Stimulation (cTBS)
Repetitive Transcranial Magnetic Stimulation (rTMS)
rTMS is the most established and widely used form of Transcranial Magnetic Stimulation (TMS). It involves delivering a series of magnetic pulses to stimulate specific brain regions, primarily targeting areas involved in mood regulation. rTMS has been extensively studied and is FDA-approved for treating major depressive disorder (MDD) and other conditions.
How rTMS Works
- Magnetic Pulses: An electromagnetic coil is placed against the scalp, delivering repetitive magnetic pulses that create small electrical currents in the brain.
- Frequency Variations:
- High-Frequency rTMS (10–20 Hz): Stimulates and increases activity in the left dorsolateral prefrontal cortex (DLPFC), which is often underactive in depression.
- Low-Frequency rTMS (1 Hz): Suppresses activity in the right DLPFC, which can be overactive in depression or anxiety.
- Session Length: A typical rTMS session lasts 20–40 minutes, and treatments are administered five days a week over 7 weeks.
rTMS Applications Beyond Depression
While primarily used for MDD, rTMS is also being explored and applied for other conditions, including:
- Obsessive-compulsive disorder (OCD): FDA-approved for targeting specific brain circuits.
- Post-traumatic stress disorder (PTSD): Improves emotional regulation and reduces intrusive thoughts.
- Chronic pain and migraines: Modulates pain perception pathways
Advantages of rTMS
- Non-Invasive and Well-Tolerated: No anesthesia or sedation is required, and patients can resume daily activities immediately after a session.
- Personalized Treatment: Advanced imaging techniques allow clinicians to pinpoint the most effective brain regions for stimulation based on individual needs.
- Proven Track Record: Decades of research and clinical trials support rTMS as a safe and effective treatment for depression.
Intermittent Theta Burst Stimulation (iTBS)
Intermittent Theta Burst Stimulation (iTBS) is a variation of Transcranial Magnetic Stimulation (TMS) that delivers rapid, patterned bursts of magnetic stimulation to enhance neural activity. Unlike its counterpart, continuous TBS (cTBS), which suppresses neural activity, iTBS is designed to stimulate and excite targeted brain regions, making it particularly useful for conditions like depression.
How iTBS Works
- Pulse Pattern: iTBS delivers three magnetic pulses at a frequency of 50 Hz, repeated every 200 milliseconds, but with intermittent breaks between bursts. This pattern mimics the brain’s natural theta rhythms, which are associated with learning, memory, and mood regulation.
- Duration: iTBS sessions typically last 3–4 minutes, making it one of the shortest forms of TMS treatment.
Target Areas
iTBS primarily targets the left dorsolateral prefrontal cortex (DLPFC), a region that is often underactive in individuals with depression. By enhancing activity in this area, iTBS helps to:
- Improve emotional regulation.
- Increase motivation.
- Enhance cognitive function.
Therapeutic Benefits of iTBS
- Activation of Neural Pathways: iTBS stimulates underactive brain regions, improving their connectivity with broader neural networks such as the Default Mode Network (DMN) and the Central Executive Network (CEN).
- Rapid Onset of Effects: Some studies suggest that iTBS can produce therapeutic effects faster than traditional rTMS, potentially leading to earlier symptom relief.
- Improved Mood and Energy: Patients often report a noticeable improvement in mood, energy, and concentration after a few sessions of iTBS.
Advantages of (iTBS)
- Efficiency: A typical iTBS session lasts only a few minutes, significantly reducing treatment time compared to standard rTMS, which can take 20–40 minutes per session.
- Comparable Effectiveness: Clinical trials have shown that iTBS is as effective as traditional high-frequency rTMS in treating major depressive disorder (MDD).
- Lower Patient Burden: The shorter session time makes iTBS a more convenient option for patients with busy schedules.
Applications Beyond Depression
While iTBS is primarily used for depression, emerging research is exploring its efficacy in treating other conditions, such as:
- Post-traumatic stress disorder (PTSD): Enhancing emotional processing and resilience.
- Cognitive impairments: Improving memory and executive function in neurological disorders.
Continuous Theta Burst Stimulation (cTBS)
Continuous Theta Burst Stimulation (cTBS) is a specialized form of Transcranial Magnetic Stimulation that delivers rapid bursts of magnetic pulses at a frequency designed to mimic natural theta brainwave rhythms. It is distinguished from other forms of TMS by its ability to suppress overactive neural activity in targeted brain regions.
How cTBS Works
- Pulse Pattern: cTBS uses short bursts of three pulses at a frequency of 50 Hz, repeated every 200 milliseconds. These bursts are administered continuously over a session lasting about 40–60 seconds, making it much faster than traditional rTMS.
- Target Areas: cTBS is often applied to the right dorsolateral prefrontal cortex (DLPFC), a region that is sometimes hyperactive in patients with depression and anxiety. By reducing activity in this area, cTBS helps balance brain networks associated with mood regulation.
Effects of cTBS
- Inhibitory Impact: The continuous nature of the stimulation creates an inhibitory effect on neural activity, which can help calm overactive regions contributing to symptoms of depression and anxiety.
- Therapeutic Goal: cTBS aims to restore a more balanced state of activity across neural networks, particularly when there is overactivation in the right DLPFC.
Advantages of cTBS
- Time Efficiency: Sessions last under a minute, making it highly practical for clinical use and improving accessibility for patients.
- Equivalent Efficacy: Research indicates that cTBS is as effective as standard rTMS for certain applications, including treating depression.
- Reduced Burden: Shorter treatment times lower the burden on both patients and providers, enabling more flexible scheduling.
By targeting overactive brain regions efficiently, cTBS represents a significant advancement in TMS technology, expanding treatment options for individuals with depression and other mental health conditions.
Summary of Research on TMS Benefits
Research highlights TMS as an effective, non-invasive treatment for major depressive disorder. Key findings include:
- Durability of effects: Studies indicate that benefits can last 12 months after completing a TMS course, ocassionally with maintenance sessions.
- Efficacy in treatment-resistant cases: Clinical trials show significant improvement in symptoms for patients who did not respond to at least two antidepressants.
- Other mental health conditions: Emerging evidence supports TMS in treating conditions like obsessive-compulsive disorder (OCD), PTSD, and anxiety disorders.
TMS’s non-invasive nature and minimal side effect profile make it a vital tool in modern psychiatry, especially for patients seeking alternatives to traditional pharmacological treatments.
Safety of TMS
TMS is considered a safe and well-tolerated treatment for depression. Unlike medications, it does not affect the whole body, reducing the risk of systemic side effects.
Common Side Effects:
- Mild headache or scalp discomfort (usually subsides after the first few sessions)
- Temporary lightheadedness
- Tingling sensation in the facial muscles
Rare Side Effects:
- Seizures (extremely rare, with an estimated risk of 1 in 30,000 treatments)
Patients undergo a thorough medical screening to minimize risks, ensuring TMS is suitable for their individual health profiles.
Typical Patient Experience of TMS
Patients often describe TMS as a comfortable and straightforward process. Here’s what a typical session involves:
- Initial Assessment: A psychiatrist maps the patient’s brain to identify the target area and determine optimal stimulation intensity.
- During the Session:
- The patient sits in a reclining chair.
- An electromagnetic coil is placed over the scalp, delivering painless magnetic pulses.
- Sessions last about 20 minutes (shorter for theta burst stimulation).
- After the Session:
- Patients can resume normal activities immediately.
- Most individuals require 5 sessions per week for 7 weeks.
Patients frequently report feeling a gradual improvement in mood, energy, and concentration within a few weeks of starting treatment.
Conclusion
Transcranial Magnetic Stimulation (TMS) represents a transformative approach to treating major depressive disorder and other mental health conditions. With its ability to target specific brain regions, a strong safety profile, and significant clinical effectiveness, TMS offers hope for individuals who have not responded to traditional treatments. If you or someone you know struggles with depression, exploring TMS with a qualified provider could be the first step toward recovery.
References
- TMS Basics and Mechanisms of Action
- George, M. S., & Post, R. M. (2011). Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. American Journal of Psychiatry, 168(4), 356–364. https://doi.org/10.1176/appi.ajp.2011.10060864
- Pascual-Leone, A., Walsh, V., & Rothwell, J. (2000). Transcranial magnetic stimulation in cognitive neuroscience: Virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology, 10(2), 232–237.
- Different Forms of TMS (rTMS, cTBS, iTBS)
- Huang, Y. Z., Edwards, M. J., Rounis, E., Bhatia, K. P., & Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45(2), 201–206. https://doi.org/10.1016/j.neuron.2004.12.033
- Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., … & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. The Lancet, 391(10131), 1683–1692. https://doi.org/10.1016/S0140-6736(18)30295-2
- Effectiveness Compared to Antidepressants
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., … & George, M. S. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208–1216. https://doi.org/10.1016/j.biopsych.2007.01.018
- Neural Networks and Connectivity in Depression
- Mayberg, H. S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin, 65(1), 193–207.
- Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124(1), 1–38. https://doi.org/10.1196/annals.1440.011
- TMS Safety and Side Effects
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., & The Safety of TMS Consensus Group. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016
- Patient Experience
- Carpenter, L. L., Janicak, P. G., Aaronson, S. T., Boyadjis, T., Brock, D. G., Cook, I. A., … & Demitrack, M. A. (2012). Transcranial magnetic stimulation (TMS) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depression and Anxiety, 29(7), 587–596. https://doi.org/10.1002/da.21969
- Advanced Applications and Emerging Research
- Noda, Y., Silverstein, W. K., Barr, M. S., Vila-Rodriguez, F., Downar, J., Rajji, T. K., & Blumberger, D. M. (2015). Transcranial magnetic stimulation in the treatment of major depressive disorder: Current status and future directions. Molecular Psychiatry, 20(6), 573–591. https://doi.org/10.1038/mp.2015.32
- TMS Targeting and Personalization
- Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., & Pascual-Leone, A. (2012). Efficacy of transcranial magnetic stimulation for depression related to intrinsic functional connectivity of the subgenual cingulate. Biological Psychiatry, 72(7), 595–603. https://doi.org/10.1016/j.biopsych.2012.04.028
This reference list provides a foundation for the evidence-based insights shared in the content above, ensuring reliability and relevance for educational or clinical use.
2. How Effective Is TMS Compared to Antidepressants?
TMS offers a compelling alternative for individuals with depression who have not achieved adequate relief from antidepressants. Studies show that up to 60-80% of patients experience significant improvement in symptoms, while around 60% achieve full remission after TMS treatment. This contrasts with antidepressants, where only about 30% of patients achieve remission on their first medication trial.
TMS effectiveness often surpasses antidepressants in treatment-resistant depression, offering hope for those who have not responded to multiple medication regimens. Additionally, TMS has fewer systemic side effects (e.g., no weight gain, sexual dysfunction, or drowsiness), making it a preferable choice for some patients.
3. The Area of the Brain TMS Targets
TMS primarily targets the dorsolateral prefrontal cortex (DLPFC)—a region involved in mood regulation, executive functioning, and emotional control. The left DLPFC is often underactive in patients with depression, while the right DLPFC may be overactive.
How TMS Works:
- Left DLPFC: High-frequency stimulation to increase activity in this underactive area.
- Right DLPFC: Low-frequency stimulation to reduce overactivity.

4. New Understanding of Major Depressive Disorder: From Chemical Imbalance to Neural Connectivity
The traditional understanding of major depressive disorder (MDD) has long focused on the “chemical imbalance” theory, emphasizing deficits in neurotransmitters such as serotonin, dopamine, and norepinephrine. While these chemical aspects play a role, emerging research reveals that MDD is more accurately characterized by disruptions in the connectivity and functioning of neural networks and pathways within the brain. This paradigm shift has profound implications for treatments like TMS.
From Chemical Imbalance to Network Dysregulation
Modern neuroscience views depression as a disorder of dysregulated brain networks rather than simply a lack of neurotransmitters. Here’s how this new understanding has developed:
- Neural Connectivity:
- In depression, key brain regions, including the prefrontal cortex, limbic system, and subcortical structures, show disrupted communication and synchronization.
- For example, overactivation of the amygdala (associated with emotional reactivity) and underactivation of the prefrontal cortex (responsible for cognitive control) create an imbalance that perpetuates negative emotional states.
- Functional Connectivity of Neural Networks:
- Research using functional MRI (fMRI) has highlighted altered connectivity in networks like the Default Mode Network (DMN), Salience Network (SN), and Central Executive Network (CEN).
- These changes lead to core symptoms of depression, such as rumination, emotional dysregulation, and cognitive impairments.
- Pathways Over Chemical Focus:
- While serotonin and other neurotransmitters remain essential, their role is now understood in the context of modulating the activity and connectivity of these networks.
- The disruption of pathways connecting the DLPFC with deeper structures like the hippocampus and amygdala is a primary target for innovative treatments like TMS.
TMS and the Connectivity Model
TMS fits seamlessly into this new understanding by directly influencing network connectivity rather than solely targeting neurotransmitter levels. Here’s how TMS works within this framework:
- Restoring Neural Balance:
- By stimulating the dorsolateral prefrontal cortex (DLPFC), TMS modulates connectivity with other brain regions, helping recalibrate overactive or underactive networks.
- Targeting Dysregulated Networks:
- TMS reduces the overactivity of the DMN, improving a patient’s ability to disengage from negative self-focus and rumination.
- It strengthens the functional communication between the DLPFC and regions of the SN and CEN, restoring cognitive control and emotional regulation.
- A Personalized Approach:
- Advanced imaging techniques (e.g., fMRI-guided TMS) allow clinicians to personalize TMS treatments by identifying the unique connectivity patterns contributing to a patient’s depression.
Broader Implications for Depression Treatment
This shift from a chemical imbalance to a connectivity-focused understanding has opened new avenues for therapeutic innovation:
- Non-invasive neuromodulation techniques like TMS directly address network dysfunctions without the systemic side effects of medications.
- It also explains why some patients with MDD may not respond to antidepressants: their underlying issues may lie more in network dysregulation than in neurotransmitter imbalances.
As the connectivity model continues to evolve, treatments like TMS are at the forefront of providing more effective and personalized care for individuals with major depressive disorder. This approach not only alleviates symptoms but also improves the underlying brain dynamics that support emotional and cognitive well-being.
5. TMS Impact on Key Neural Networks
In addition to directly targeting the dorsolateral prefrontal cortex (DLPFC), Transcranial Magnetic Stimulation (TMS) influences several interconnected neural networks that play critical roles in mood regulation, cognitive function, and emotional processing. The three most relevant networks affected by TMS are the Default Mode Network (DMN), the Salience Network (SN), and the Central Executive Network (CEN).
1. Default Mode Network (DMN)
The Default Mode Network is active during rest and self-referential thinking, such as daydreaming, introspection, or rumination. In individuals with depression, the DMN is often hyperactive, contributing to excessive negative self-focus and rumination.
- TMS Impact on the DMN:
- High-frequency stimulation of the left DLPFC disrupts the overactivity of the DMN, reducing rumination and improving emotional regulation.
- This recalibration helps patients shift focus from self-critical thoughts to more adaptive cognitive processes, facilitating recovery from depressive symptoms.
2. Salience Network (SN)
The Salience Network detects and filters relevant stimuli, determining what requires attention. It plays a central role in emotional regulation and switching between the DMN and the CEN. Dysfunction in the SN is associated with difficulties in emotional processing and heightened sensitivity to negative stimuli, common in depression.
- TMS Impact on the SN:
- TMS enhances SN functioning by improving communication between the DLPFC and deeper brain structures like the anterior cingulate cortex (ACC), (which is associated with our will power) and insula.
- This leads to better regulation of emotional salience, helping individuals focus on positive or neutral stimuli rather than overemphasizing negative experiences.
3. Central Executive Network (CEN)
The Central Executive Network is involved in higher-order cognitive processes, including working memory, decision-making, and goal-oriented behavior. Depression often results in underactivity of the CEN, leading to poor focus, indecisiveness, and diminished problem-solving abilities.
- TMS Impact on the CEN:
- By stimulating the left DLPFC, TMS increases the activity and connectivity within the CEN, enhancing cognitive control and executive functioning.
- Patients often report improvements in concentration, task efficiency, and the ability to plan and execute actions.
The Interplay Between Networks
TMS promotes a healthy balance and communication between these networks, enabling:
- Reduced overactivity of the DMN (less rumination).
- Improved salience processing via the SN (better emotional regulation).
- Enhanced cognitive control through the CEN (greater focus and decision-making).
This integrated effect is one of the reasons TMS is effective not only in alleviating depressive symptoms but also in addressing the cognitive and emotional challenges that often accompany depression.
6. Different Forms of TMS
There are several variations of TMS, tailored to meet different clinical needs, these include the following forms of TMS:
- Repetitive Transcranial Magnetic Stimulation (rTMS)
- Intermittent Theta Burst Stimulation (iTBS)
- Continuous Theta Burst Stimulation (cTBS)
1. Repetitive Transcranial Magnetic Stimulation (rTMS)
Repetitive Transcranial Magnetic Stimulation (rTMS) is the most established and widely used form of Transcranial Magnetic Stimulation (TMS). It involves delivering a series of magnetic pulses to stimulate specific brain regions, primarily targeting areas involved in mood regulation. rTMS has been extensively studied and is FDA-approved for treating major depressive disorder (MDD) and other conditions.
How rTMS Works
- Magnetic Pulses: An electromagnetic coil is placed against the scalp, delivering repetitive magnetic pulses that create small electrical currents in the brain.
- Frequency Variations:
- High-Frequency rTMS (10–20 Hz): Stimulates and increases activity in the left dorsolateral prefrontal cortex (DLPFC), which is often underactive in depression.
- Low-Frequency rTMS (1 Hz): Suppresses activity in the right DLPFC, which can be overactive in depression or anxiety.
- Session Length: A typical rTMS session lasts 20–40 minutes, and treatments are administered five days a week over 7 weeks.
Target Brain Regions
rTMS focuses on the dorsolateral prefrontal cortex (DLPFC), a region critical for:
- Mood regulation.
- Decision-making.
- Emotional processing.
Stimulation of the DLPFC improves its connectivity with other brain regions, including the amygdala, hippocampus, and anterior cingulate cortex, which are involved in emotion and memory processing.
Therapeutic Benefits of rTMS
- Effective for Treatment-Resistant Depression:
- Approximately 60-80% of patients experience significant symptom improvement.
- About 60% achieve remission one year after completing a treatment course.
- Minimal Side Effects:
- Unlike medications, rTMS does not cause systemic side effects like weight gain or drowsiness.
- Long-Lasting Effects:
- Studies indicate that the benefits of rTMS can last for up to 12 months with maintenance sessions.
Applications Beyond Depression
While primarily used for MDD, rTMS is also being explored and applied for other conditions, including:
- Obsessive-compulsive disorder (OCD): FDA-approved for targeting specific brain circuits.
- Post-traumatic stress disorder (PTSD): Improves emotional regulation and reduces intrusive thoughts.
- Chronic pain and migraines: Modulates pain perception pathways.
Advantages of rTMS
- Non-Invasive and Well-Tolerated: No anesthesia or sedation is required, and patients can resume daily activities immediately after a session.
- Personalized Treatment: Advanced imaging techniques allow clinicians to pinpoint the most effective brain regions for stimulation based on individual needs.
- Proven Track Record: Decades of research and clinical trials support rTMS as a safe and effective treatment for depression.
Intermittent Theta Burst Stimulation (iTBS)
Intermittent Theta Burst Stimulation (iTBS) is a variation of Transcranial Magnetic Stimulation (TMS) that delivers rapid, patterned bursts of magnetic stimulation to enhance neural activity. Unlike its counterpart, continuous TBS (cTBS), which suppresses neural activity, iTBS is designed to stimulate and excite targeted brain regions, making it particularly useful for conditions like depression.
How iTBS Works
- Pulse Pattern: iTBS delivers three magnetic pulses at a frequency of 50 Hz, repeated every 200 milliseconds, but with intermittent breaks between bursts. This pattern mimics the brain’s natural theta rhythms, which are associated with learning, memory, and mood regulation.
- Duration: iTBS sessions typically last 3–4 minutes, making it one of the shortest forms of TMS treatment.
Target Areas
iTBS primarily targets the left dorsolateral prefrontal cortex (DLPFC), a region that is often underactive in individuals with depression. By enhancing activity in this area, iTBS helps to:
- Improve emotional regulation.
- Increase motivation.
- Enhance cognitive function.
Therapeutic Benefits of iTBS
- Activation of Neural Pathways: iTBS stimulates underactive brain regions, improving their connectivity with broader neural networks such as the Default Mode Network (DMN) and the Central Executive Network (CEN).
- Rapid Onset of Effects: Some studies suggest that iTBS can produce therapeutic effects faster than traditional rTMS, potentially leading to earlier symptom relief.
- Improved Mood and Energy: Patients often report a noticeable improvement in mood, energy, and concentration after a few sessions of iTBS.
Advantages of iTBS
- Efficiency: A typical iTBS session lasts only a few minutes, significantly reducing treatment time compared to standard rTMS, which can take 20–40 minutes per session.
- Comparable Effectiveness: Clinical trials have shown that iTBS is as effective as traditional high-frequency rTMS in treating major depressive disorder (MDD).
- Lower Patient Burden: The shorter session time makes iTBS a more convenient option for patients with busy schedules.
Applications Beyond Depression
While iTBS is primarily used for depression, emerging research is exploring its efficacy in treating other conditions, such as:
- Post-traumatic stress disorder (PTSD): Enhancing emotional processing and resilience.
- Cognitive impairments: Improving memory and executive function in neurological disorders.
Continuous Theta Burst Stimulation (cTBS)
Continuous Theta Burst Stimulation (cTBS) is a specialized form of Transcranial Magnetic Stimulation that delivers rapid bursts of magnetic pulses at a frequency designed to mimic natural theta brainwave rhythms. It is distinguished from other forms of TMS by its ability to suppress overactive neural activity in targeted brain regions.
How cTBS Works
- Pulse Pattern: cTBS uses short bursts of three pulses at a frequency of 50 Hz, repeated every 200 milliseconds. These bursts are administered continuously over a session lasting about 40–60 seconds, making it much faster than traditional rTMS.
- Target Areas: cTBS is often applied to the right dorsolateral prefrontal cortex (DLPFC), a region that is sometimes hyperactive in patients with depression and anxiety. By reducing activity in this area, cTBS helps balance brain networks associated with mood regulation.
Effects of cTBS
- Inhibitory Impact: The continuous nature of the stimulation creates an inhibitory effect on neural activity, which can help calm overactive regions contributing to symptoms of depression and anxiety.
- Therapeutic Goal: cTBS aims to restore a more balanced state of activity across neural networks, particularly when there is overactivation in the right DLPFC.
Advantages of cTBS
- Time Efficiency: Sessions last under a minute, making it highly practical for clinical use and improving accessibility for patients.
- Equivalent Efficacy: Research indicates that cTBS is as effective as standard rTMS for certain applications, including treating depression.
- Reduced Burden: Shorter treatment times lower the burden on both patients and providers, enabling more flexible scheduling.
By targeting overactive brain regions efficiently, cTBS represents a significant advancement in TMS technology, expanding treatment options for individuals with depression and other mental health conditions.
7. Summary of Research on TMS Benefits
Research highlights TMS as an effective, non-invasive treatment for major depressive disorder. Key findings include:
- Durability of effects: Studies indicate that benefits can last 12 months after completing a TMS course, ocassionally with maintenance sessions.
- Efficacy in treatment-resistant cases: Clinical trials show significant improvement in symptoms for patients who did not respond to at least two antidepressants.
- Other mental health conditions: Emerging evidence supports TMS in treating conditions like obsessive-compulsive disorder (OCD), PTSD, and anxiety disorders.
TMS’s non-invasive nature and minimal side effect profile make it a vital tool in modern psychiatry, especially for patients seeking alternatives to traditional pharmacological treatments.
8. Safety of TMS
TMS is considered a safe and well-tolerated treatment for depression. Unlike medications, it does not affect the whole body, reducing the risk of systemic side effects.
Common Side Effects:
- Mild headache or scalp discomfort (usually subsides after the first few sessions)
- Temporary lightheadedness
- Tingling sensation in the facial muscles
Rare Side Effects:
- Seizures (extremely rare, with an estimated risk of 1 in 30,000 treatments)
Patients undergo a thorough medical screening to minimize risks, ensuring TMS is suitable for their individual health profiles.
9. Typical Patient Experience of TMS
Patients often describe TMS as a comfortable and straightforward process. Here’s what a typical session involves:
- Initial Assessment: A psychiatrist maps the patient’s brain to identify the target area and determine optimal stimulation intensity.
- During the Session:
- The patient sits in a reclining chair.
- An electromagnetic coil is placed over the scalp, delivering painless magnetic pulses.
- Sessions last about 20 minutes (shorter for theta burst stimulation).
- After the Session:
- Patients can resume normal activities immediately.
- Most individuals require 5 sessions per week for 7 weeks.
Patients frequently report feeling a gradual improvement in mood, energy, and concentration within a few weeks of starting treatment.
10. Conclusion
Transcranial Magnetic Stimulation (TMS) represents a transformative approach to treating major depressive disorder and other mental health conditions. With its ability to target specific brain regions, a strong safety profile, and significant clinical effectiveness, TMS offers hope for individuals who have not responded to traditional treatments. If you or someone you know struggles with depression, exploring TMS with a qualified provider could be the first step toward recovery.



